
A paper on high-pressure forms of chlorine claims 2] Some of these elements (including hydrogen, oxygen, nitrogen, chlorine, and xenon) solidify and form metallic phases when under sufficient pressure. This includes hydrogen, oxygen, nitrogen, the halogens up to chlorine and the noble gases up to radon. If an element is in the gas phase, we typically don't describe it as a metal. How on earth is it possible for 92 of the 118 discovered elements to be metals!? The absence of molecules and the abundance of nearest neighbors gives rise to the band structure of metals (and the delocalisation of electrons) that explains many of their properties. The former seems more "natural", given that the atoms are all of the same type. Either the atoms will interact with each other in the same way (like in a metal), or they will form groups of strongly interacting atoms (called molecules) interacting less strongly with other groups (like liquid bromine or solid iodine). In a solid or liquid, the atoms will be close together in an element, they will all be the same. So to be considered a metal, an element has to be in a condensed physical state. While the OP's question is about elements, alloys are classified as metals as well, even when they contain some non-metalls like the carbon in steel. Mostly, we think of metals as solids, although the liquid mercury is commonly defined as metal as well. I think it's possible to argue that metallicity (or more generally, delocalized bonding) is more natural, and it's the non-metals which are unusual - why do we have a bunch of those? Definition and structure of metals I will use this excellent comment as a starting point: In all these elements, the outer shells are somewhat similar, but the increasing size of that outer shell make them more metallic. Polonium is a metal, conductive (0.40 µΩ⋅m at 0 ☌) and shiny (and incredibly dangerous).
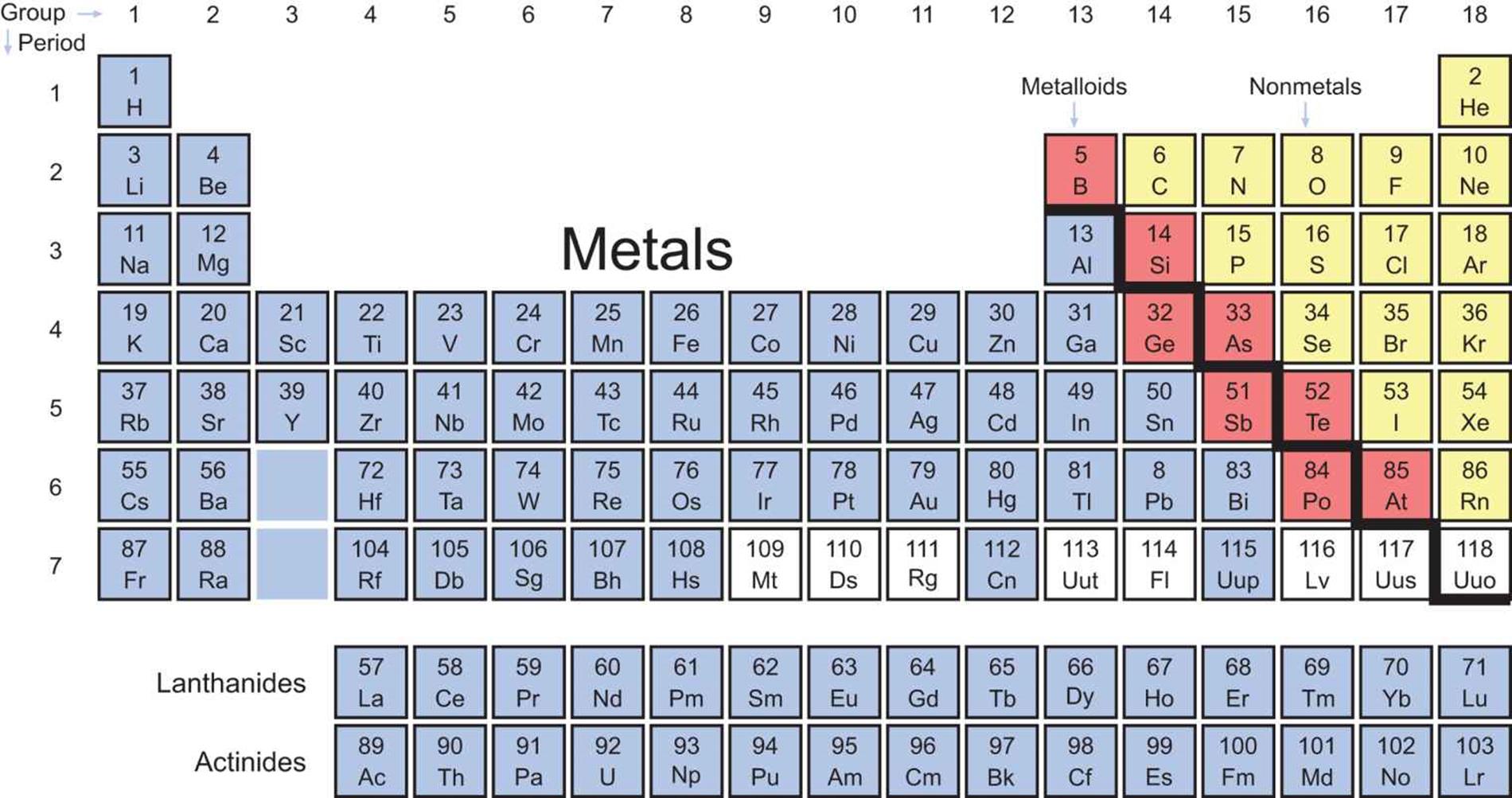
Tellurium is also a semimetal, more conductive than Se.Clearly, the outer electrons are not firmly attached. Selenium is a metalloid, shiny in some allotropes, and conducting electricity with the help of a photon or two.


 0 kommentar(er)
0 kommentar(er)
